Cold Chain Vaccine Monitoring
WHO-Compliant Vaccine Temperature Monitoring
Vaccines can save lives—if kept within strict temperature ranges from production to administration. Temperature excursions can compromise potency and patient safety. ELPRO provides temperature monitoring solutions that meet World Health Organization (WHO) standards, supporting safe storage and transport worldwide. From first-mile manufacturing to last-mile delivery in remote clinics, ELPRO devices help ensure compliance, visibility, and accountability.
- WHO-prequalified data loggers for vaccine cold chain compliance
- Easy-to-use, single-use devices for clear status indication
- End-to-end temperature monitoring with automatic data capture and alerts
- Fully compliant documentation to meet WHO, UNICEF and NGO audit requirements
Patient Safety Starts with Cold Chain Integrity
Whether manufacturing, transporting or administering vaccines, stakeholders across the supply chain face strict requirements to maintain product stability. ELPRO offers monitoring services, software and WHO-prequalified data loggers that help ensure vaccine safety and compliance—no matter how complex the logistics.
-
NGOs & Global Health Partners
-
Logistics Providers
-
Healthcare Facilities & Clinics
Support Safe Vaccine Access in Challenging Environments
WHO-prequalified LIBERO CB and CS devices give health programs peace of mind during remote immunization campaigns. Easy-to-use indicators and reliable data loggers ensure vaccines remain within safe temperature ranges—supporting compliance, minimizing waste, and protecting patients in low-resource settings.
Maintain Oversight During Complex Global Shipments
ELPRO’s cold chain solutions help logistics teams monitor temperature-critical vaccine shipments. LIBERO PDF data loggers provide automated temperature monitoring reports that can be sent by e-mail while ensuring GDP and IATA compliance at every transport leg of the journey.
If you are looking for real-time visibility and automated alerts, ELPRO provides LIBERO Gx real-time IoT data loggers for global connectivity.

Protect Vaccine Potency at the Point of Care
ELPRO’s stationary monitoring systems track refrigerators, freezers, and ambient storage zones 24/7. Instant alarms and automatic reporting help clinics and pharmacies being prepared for upcoming audits while maintaining cold chain compliance—even during weekends, holidays or unexpected power outages.
Comprehensive Monitoring Solutions for Vaccine Cold Chains
ELPRO offers temperature monitoring solutions tailored to the unique challenges of vaccine storage and distribution. From WHO-prequalified data loggers to cloud-based software platforms, our systems ensure vaccines remain within safe temperature ranges at every point in the supply chain.
- WHO-prequalified data loggers for vaccine compliance
- Intuitive software with clear data visualization
- Automated notifications, alarm and reporting
- API integration for seamless data transfer to other systems
- Guaranteed GxP-compliant solutions


Single-Use
- WHO prequalified: PQS E006/022
- 2 alarm limits
- Operating range: -30 °C to +70 °C
- Download WHO data sheet

Single-Use Multi-Level
- WHO prequalified: PQS E006/023
- 8 alarm zones
- Operating range: -30 °C to +70 °C
- Download WHO data sheet
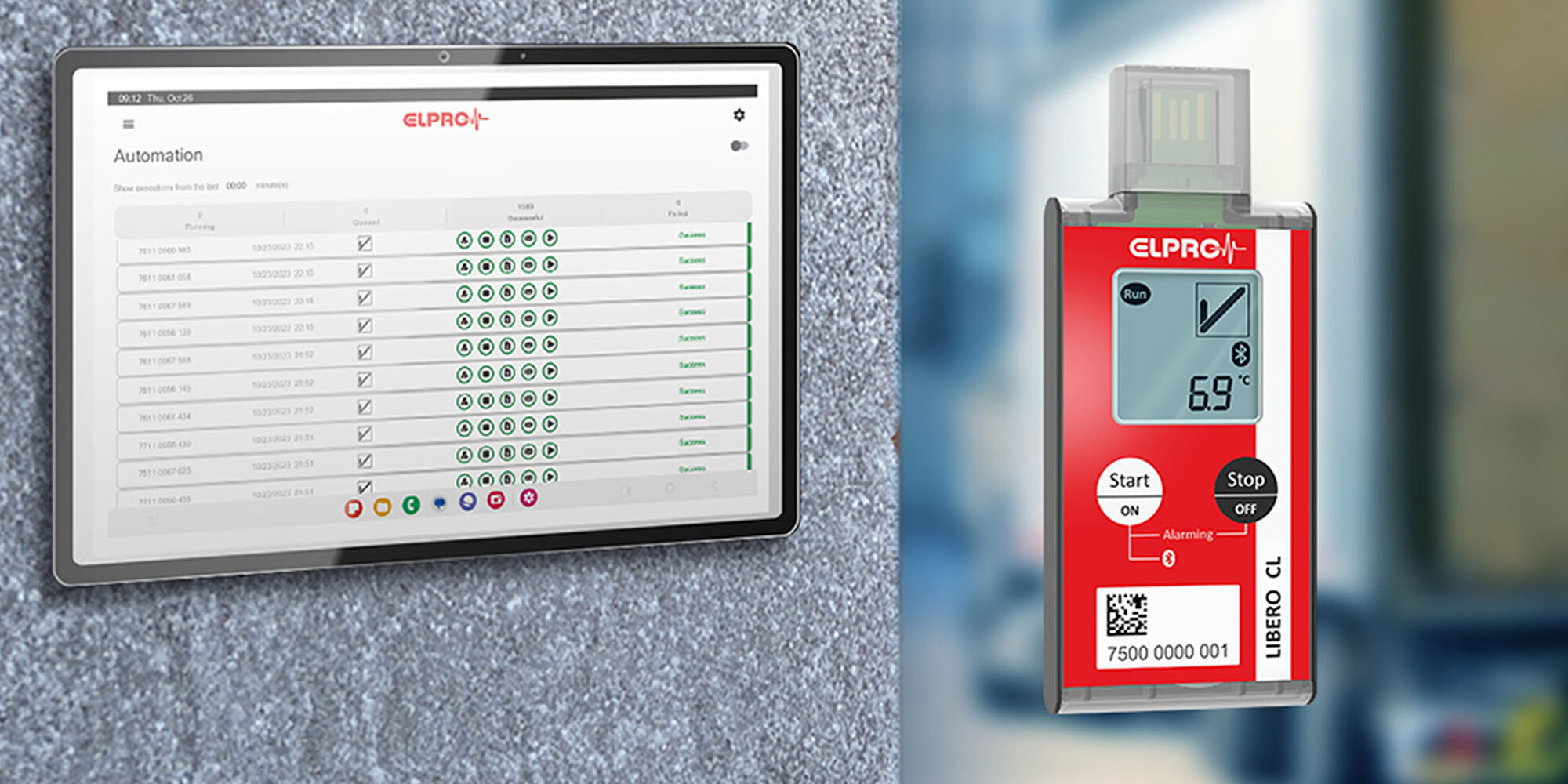
Multi-Use Bluetooth®
- WHO prequalification pending
- 8 alarm zones
- Operating range: -30 °C to +70 °C

Single-Use Dry Ice
- Temperature down to -95 °C
- Direct dry ice placement
- Automatic PDF report
- Detailed data analysis

Multi-Use Cryo
- -200 °C to +400 °C
- Strong M8 connector
- USB or Bluetooth® connection
- Alkaline battery—IATA-compliant
Equipment Monitoring for Every Temperature Range
Vaccines remain effective only when stored within the correct temperature range—not just during transport, but also in storage at manufacturing sites, distribution hubs, and healthcare facilities. In refrigerators, freezers, or ultra-low freezers, reliable hardware and software solutions help maintain compliance, ensure temperature visibility, and support full accountability.
“In projects like this, where time is of the essence and we rely heavily on the expertise of the supplier, especially soft factors play a major role in addition to meeting user requirements. ELPRO already convinced us from the very first discussions with their ready willingness to find solutions, their competence, reliability and openness.”
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk Cold Chain Distribution. Contact Us Today.

Common Questions About Vaccine Cold Chain Monitoring
Navigating vaccine logistics requires precision, compliance and reliability. Below are answers to some of the most common questions about how ELPRO’s monitoring solutions support safe, effective vaccine storage and transport.What makes ELPRO’s solutions ideal for vaccine cold chain monitoring?
ELPRO offers end-to-end temperature monitoring solutions tailored specifically for vaccines—from WHO-prequalified data loggers to real-time cloud software. Our systems provide automated compliance, detailed reporting and flexible monitoring options to ensure vaccine potency throughout storage and transit.
Are ELPRO data loggers WHO prequalified?
Yes, some ELPRO data loggers are WHO prequalified, including the LIBERO CB (PQS E006/022) and LIBERO CS (PQS E006/023). These devices meet rigorous PQS standards and are trusted by global health organizations and NGOs for reliable vaccine monitoring in low-resource settings.
What WHO guidelines apply to vaccine temperature monitoring?
The World Health Organization outlines specific performance, accuracy and durability requirements for vaccine temperature monitoring devices under the Performance, Quality and Safety (PQS) system. Devices must demonstrate reliable performance under field conditions and ensure that vaccines are stored between +2 °C and +8 °C unless otherwise specified. ELPRO’s WHO-prequalified solutions are designed in accordance with these PQS guidelines.
How do I choose the right ELPRO monitoring device for my vaccine cold chain?
Device selection depends on your application: For last-mile vaccine delivery or remote field campaigns, single-use WHO-prequalified loggers like LIBERO CB or CS are ideal. For real-time tracking during global shipments, consider LIBERO Gx. For clinics or storage sites, multi-use loggers with Bluetooth® and elproCLOUD integration offer continuous visibility. ELPRO can help assess your use and recommend the right solution.
Can I monitor vaccine temperatures in real time?
Yes, ELPRO offers real-time monitoring solutions using cellular and Bluetooth® data loggers integrated with elproCLOUD and liberoMANAGER platforms. This allows for automatic alerts, immediate visibility and fast decision-making to protect against temperature excursions during transit.
How do ELPRO systems support compliance and audits?
ELPRO’s software solutions provide 21 CFR Part 11–compliant digital records, automatic reporting and clear audit trails. Whether you use our single-use loggers or install permanent systems, you’ll have easy access to the data and documentation needed for regulatory inspections.
Do ELPRO solutions work in remote or low-infrastructure environments?
Yes. ELPRO’s battery-powered, compact data loggers are easy to deploy anywhere—no specialized training or infrastructure required. WHO-prequalified options like the LIBERO CB and CS are especially well-suited for vaccine campaigns in remote or resource-limited areas.
What support does ELPRO offer for implementation?
ELPRO provides expert consultation, validation support, and ongoing technical assistance to help configure and maintain your monitoring system. Whether for a centralized facility or international distribution network, we tailor our service to your specific needs.
Can I integrate ELPRO data with other systems?
Yes. Both elproCLOUD and liberoMANAGER offer API integrations, making it easy to share environmental monitoring data with ERP, LIMS or other enterprise systems. This ensures smooth data flow and centralized oversight.
Is ELPRO monitoring scalable for large vaccine programs?
Yes, ELPRO offers solutions are modular and scalable—ideal for small clinics, national immunization programs or multi-national pharmaceutical supply chains. From simple indicators to multi-site cloud platforms, our systems grow with your needs.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.