Clinical Trials
Clinical Trials Temperature Monitoring Systems for Every Phase
Clinical trial shipments leave no room for uncertainty. From complex, mixed-load shipments to sensitive high-risk personalized medicines and therapies, monitoring solutions must ensure compliance, traceability, visibility and protection.
- Qualified monitoring systems for products during transport and on-site storage
- Data loggers for every pharmaceutical application
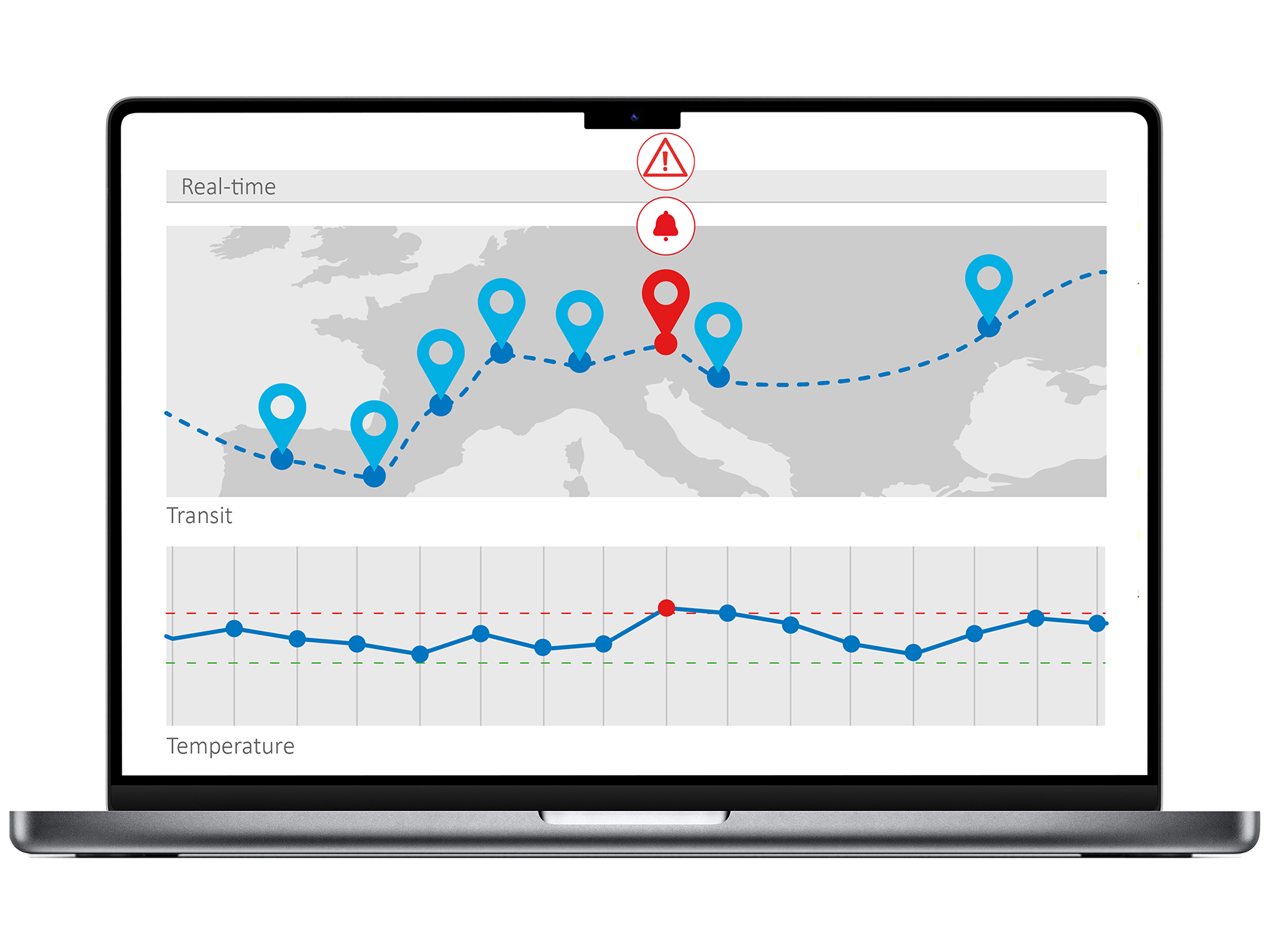
- Real-time alarms and notifications
- API integration for seamless data transfer to your clinical trial monitoring platform
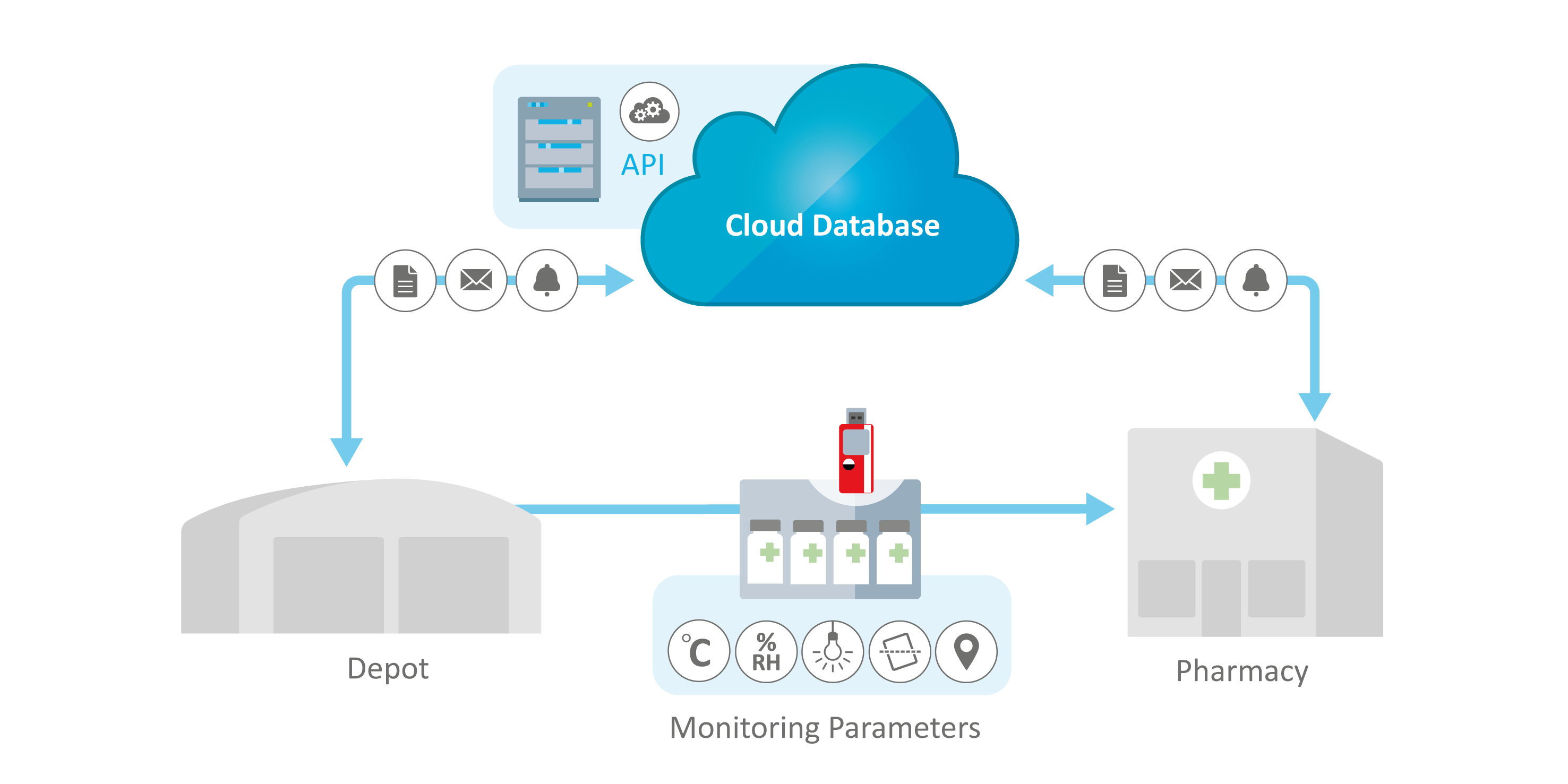
Clinical Trials Monitoring System Applications
ELPRO-qualified monitoring systems for clinical trials protect bulk supplies during transit, ensuring their safe and timely delivery to trial sites. They also support precise on-site storage monitoring to maintain the integrity and quality of supplies—while enabling efficient direct-to-patient distribution.
-
Monitoring During Transit
-
On-Site Storage Monitoring
-
Direct-to-Patient

Bulk Supplies to Various Depots and Clinical Sites
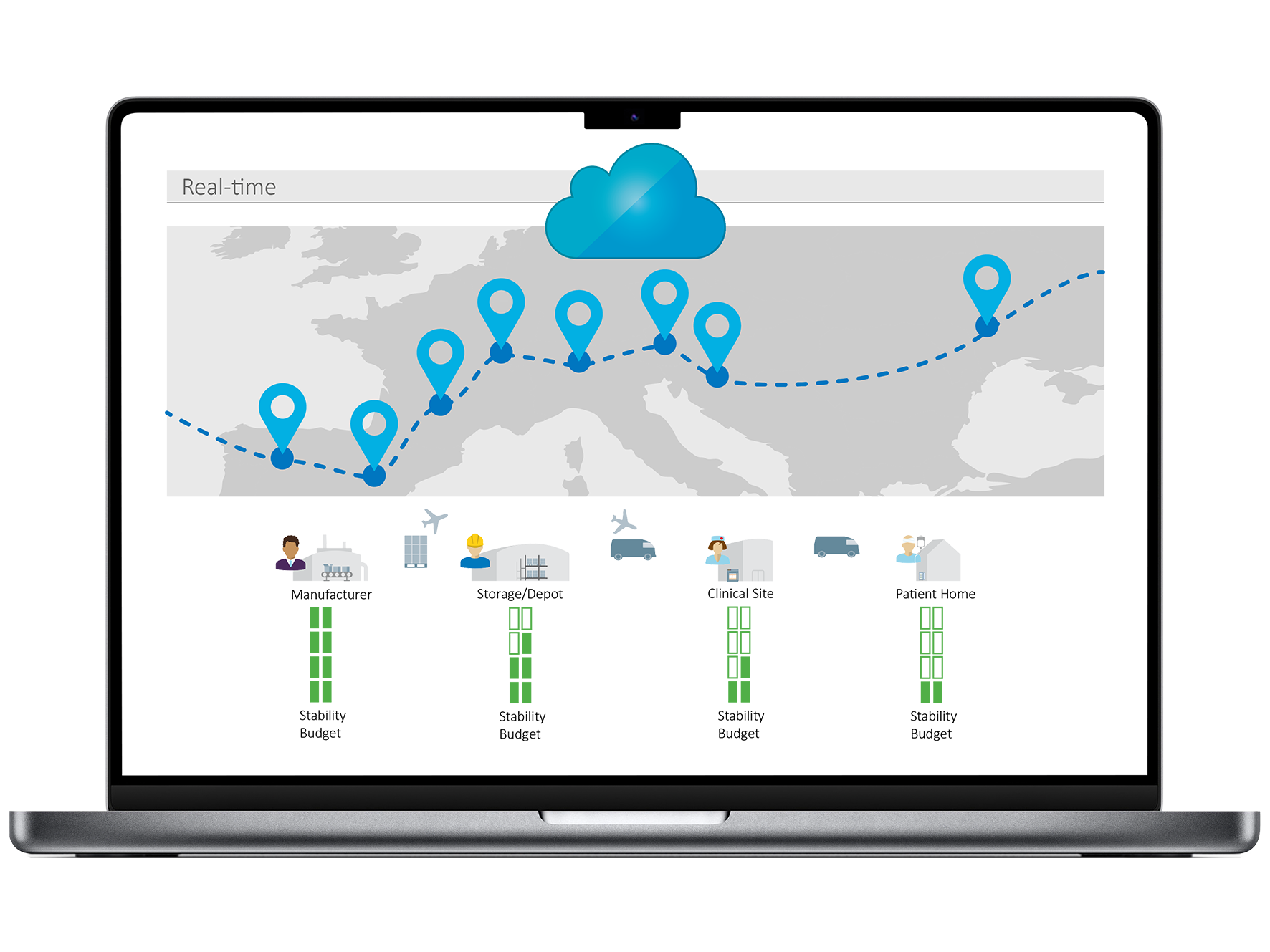
Investigational medicinal products (IMPs) often follow a complex path from manufacturer to patient—typically via contract manufacturers and regional depots across multiple countries before reaching the clinical site. Careful planning of this multi-step supply chain is essential to ensure timely delivery, product integrity, and minimal waste.
From global mixed-load shipments to temporary storage in internationally distributed depots, ELPRO safeguards IMPs, monitors product integrity and enables faster release times with continuous real-time data, predictive analytics, automated reporting, and dynamic stability budget management.

Safeguarding IMP Stability from Depot to Clinical Site
Temperature monitoring of IMPs is essential not only during transport but also while stored at depots and clinical sites throughout the study. This is particularly important for sensitive products such as vaccines, insulin, blood products, antibiotics, monoclonal antibodies, growth hormones, cancer treatment drugs, eye medications, and personalized or precision medicines.
Monitoring that IMPs remain within specified temperature ranges helps preserve their stability and efficacy, supports patient safety, and provides documented proof of proper handling—forming a vital part of quality assurance in clinical trials.

Visibility from Site to Home
Direct-to-patient deliveries are becoming increasingly important in clinical trials to improve patient access and convenience. However, monitoring the temperature of IMPs throughout this extended supply chain—from central depots to the patient’s home—remains a challenge.
One highly effective solution is the use of kit-level indicators such as the LIBERO ITS. This electronic, multi-level indicator is attached directly to each clinical trial kit, continuously monitoring temperature and calculating the remaining stability budget from packaging through to final use. Its simple green/red LED status display makes it easy for patients to understand, ensuring safe use and compliance over the full shelf life of up to four years.
Solutions for Every Study Phase
Hardware and software solutions from ELPRO help maintain quality and compliance across all phases of your clinical study. They provide faster, reliable, actionable data that empowers smarter decisions, real-time accountability, automated workflows, and ensure regulatory compliance at every study milestone
- Data loggers for every pharmaceutical application
- Intuitive software with clear data visualization
- Customizable workflows
- Advanced user and access management
- Real-time alarms and notifications
- Automated reporting
- Auto assessments and software-based alarming for mixed-load shipments
- API integration for seamless data transfer to your clinical trial monitoring platform


Single-Use USB
- Temperature
- Automated PDF report generation
- USB connection

Cellular IoT Real-Time
- Temperature, humidity and position
- Real-time data generation and alarming
- Single-Use / Multi-Use

Cellular IoT Real-Time
- °C, %RH
- LTE-M and NB-IoT (global roaming)
- IT-independent installation
- Easily scalable

Smart Digital Indicator
- Strict temperature profiles
- Visual alarm status
- Thin, small and self-adhesive
- LED Status for Go/No-Go
liberoMANAGER has enabled transparency across our organization. With critical temperature data in the cloud, our supply chain teams across global sites can access the information for transport validations and make informed decisions for routes they are booking. We can foresee problems better and make informed decisions to protect our products.
Integrated Monitoring for CROs, CMOs & CDMOs
ELPRO's environmental monitoring solutions streamline your processes, safeguard critical data for your customers and positively impact your bottom line. Explore these benefits and contact us to discuss setting up a pilot program today.
Streamline Site Operations
- Empower sites with easy self-installation
- Eliminate complex setup procedures
- Reduce site staff training time
- Ensure your commitment to quality
Strengthen Quality Control
- Monitor storage units on a central platform
- Ensure GMP/GDP Complilance
- Receive instant alerts for excursions
- Maintain full audit trails automatically
Gain Total Visibility
- Access real-time data across all sites
- Track sensors and battery levels
- Get custom alerts and analytics
- Manage user groups and permissions
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk CRT Monitoring. Contact Us Today.

5 Critical Questions for Monitoring Clinical Supplies Shipments
Understanding these aspects is crucial for selecting an environmental monitoring solution that will maintain compliance, protect product integrity, and ensure patient safety throughout the clinical distribution journey.
What about regulatory compliance and validation status?
When evaluating environmental monitoring solutions, is it imperative to ask about the solution's current compliance status with FDA 21 CFR Part 11, EU GMP Annex 11, and other relevant regulatory requirements. In addition, determine if the provider can provide documentation of successful system validation in similar clinical trial environments.
Rationale:
- It's essential for ensuring data integrity and audit readiness
- You must verify electronic records and signatures meet regulatory standards
- You need evidence of successful implementations in comparable settings
- It is important to understand the validation documentation provided
- it is critical for maintaining GMP/GLP compliance throughout the trial
Do I have end-to-end temperature monitoring capabilities?
Determine full end-to-end visibility capabilities to provide continuous temperature monitoring across all critical phases—from packaging through patient delivery—and what are the specific capabilities for each phase.
Rationale:
- You must ensure no gaps in temperature monitoring
- You need to understand monitoring capabilities during transitions
- It is important to receive and verify real-time alerting capabilities
- It is critical to confirm monitoring precision at each stage
- It is essential for maintaining product quality throughout your clinical supply chain
What are data management and integration options?
It is imperative to always determine up front who owns the data that is stored in a particular system. With ELPRO, your data are always your data. Ask your potential solution provider about the system's data management capabilities, including data security, backup procedures, and the ability to integrate with existing clinical trial management systems (CTMS) and quality management systems (QMS).
Rationale:
- It is critical for maintaining data integrity
- You must ensure proper audit trail functionality for compliance
- You need to understand data access controls and permissions
- It is important to verify backup and recovery procedures
- If you need all your data in one CTMS platform, it is essential for seamless integration.
What es the alert management and response system?
There is a lot riding on local and remote alarm notifications. Slow excursion alerts and subsequent slow response times have the potential to result in the loss of millions in valuable supplies and kits, destroy irreplaceable research, or completely derail promising clinical trials. Determine how your temperature/humidity excursion alert management system will function, including escalation procedures, notification methods, and response time guarantees.
Rationale:
- It is critical for immediate response to temperature and humidity excursions
- Must understand alert customization capabilities
- You need to verify multiple notification methods
- It is important to confirm escalation procedures
- It is essential for preventing product loss and ensuring patient safety
What about system reliability and support?
Determine if your environmental monitoring solution has a proven record of reliability, including hardware failure rates, battery life for monitoring devices, and your support structure for both routine and critical situations.
Rationale:
- It is critical for ensuring continuous monitoring
- You must understand maintenance requirements
- You need to verify backup systems and redundancies
- It is important to confirm system support availability and response times
- It is essential for preventing monitoring data gaps and system failures
Other considerations
Other important considerations to think about when monitoring your clinical trial supplies and research
- Detailed technical specifications
- Case studies from similar implementations
- References from other clinical trial organizations
- Pricing models and total cost of ownership analysis
- Implementation timelines and resource requirements
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


