Cold Chain Logistics
Environmental Monitoring in Pharma Cold Chain Logistics
As the cold chain ecosystems evolve, validated monitoring solutions throughout the distribution process play a vital role. Maintain the integrity of your temperature-sensitive pharmaceuticals, vaccines, precision medicines, tissue samples, and medical devices with:
- Real-time continuous precision monitoring
- Wide range of parameters (temperature, humidity, light, tilt, and position)
- Comprehensive hardware, software and service solutions for global regulatory compliance
- Auto-assessment and software-based alarming for accelerated assessments and product releases
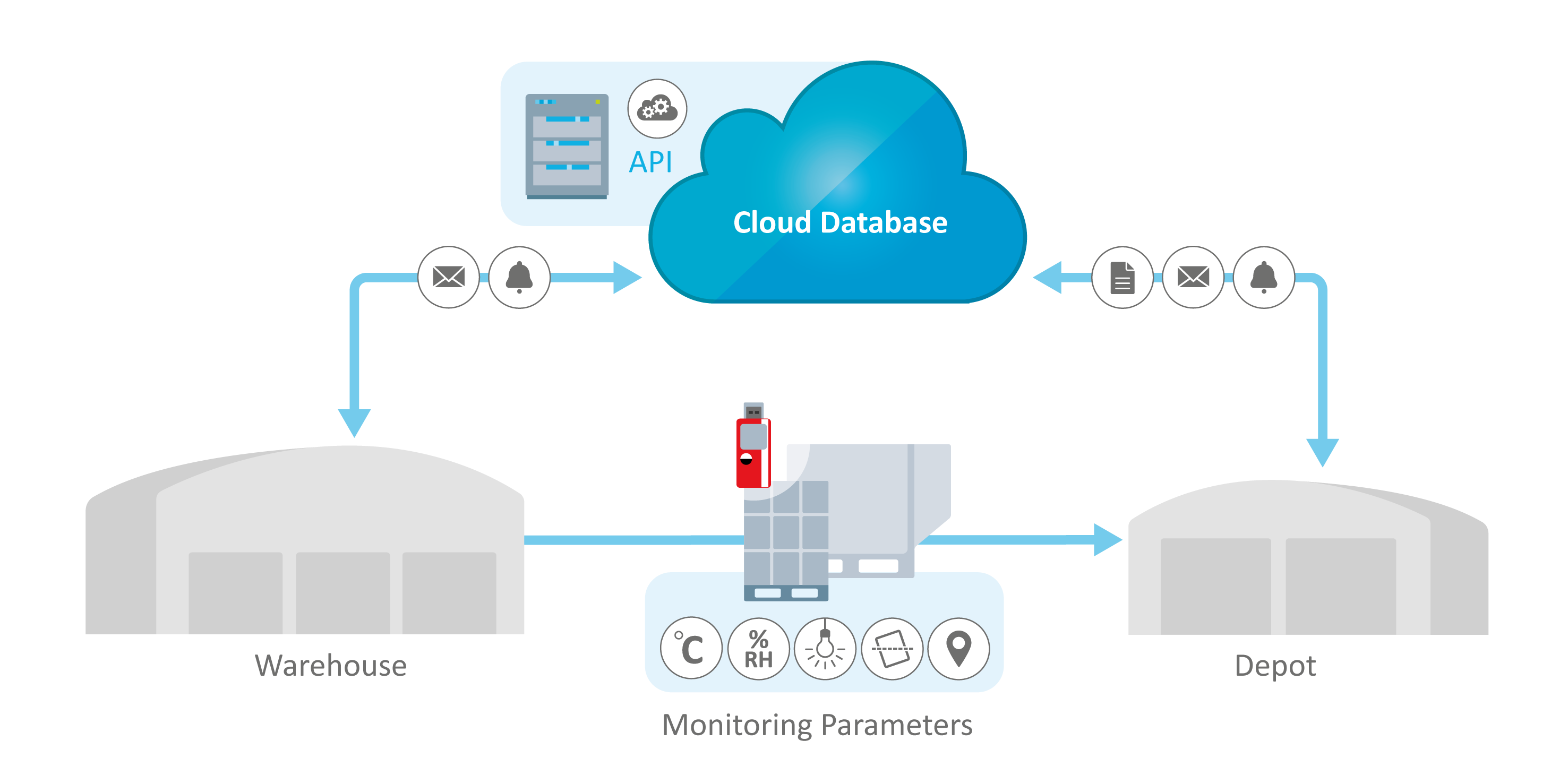
Cold Chain Pharmaceutical Logistics Products Need Protection
For over three decades, ELPRO has built a deep understanding of your cold supply chains, how to mitigate risk within them, and how to protect product efficacy, process efficiency and compliance throughout the journey in your cold chain applications:
-
Commercial
-
Clinical Trials
-
Precision/Personalized Medicine

Commercial Pharma Cold Chain Monitoring
- Monitoring of validated cold storage facilities (-80 °C to +25 °C) with backup systems
- Continuous environmental monitoring during transport
- Monitoring of thermal packaging qualified for specific temperature ranges
- Real-time deviation alerts with intervention protocols
- Temperature verification documentation

Clinical Trials Pharma Monitoring
- Continuous monitoring with validated devices
- Qualified monitoring of thermal packaging
- Temperature mapping services for storages and equipment
- Validated shipping routes with minimal handoffs
- Real-time temperature tracking with excursion alerts
Precision Medicine Cold Chain
- Validated continuous monitoring devices meeting regulatory standards
- Qualified monitoring of thermal packaging designed for sensitive personalized treatments
- Temperature mapping services for storages and equipment
- Data for optimized shipping routes minimizing handoffs for irreplaceable samples
- Real-time alerts that enable rapid interventions instead of retrospective assessments
Smart Solutions for Complex Supply Chains
Hardware and software solutions from ELPRO help maintain quality and compliance throughout the pharmaceutical and life science supply chain. Designed to simplify complex processes, they support faster, well-informed decision-making—from production to delivery.
- Data loggers for every pharma application
- Intuitive software with clear data visualization
- Customizable workflows
- Advanced user / access management
- Real-time alarms and notifications
- Automated reporting
- Auto assessments and software-based alarming for mixed-load shipments
- API integration for seamless data transfer to other systems


Single-Use USB
- Temperature
- Automated PDF report generation
- USB connection
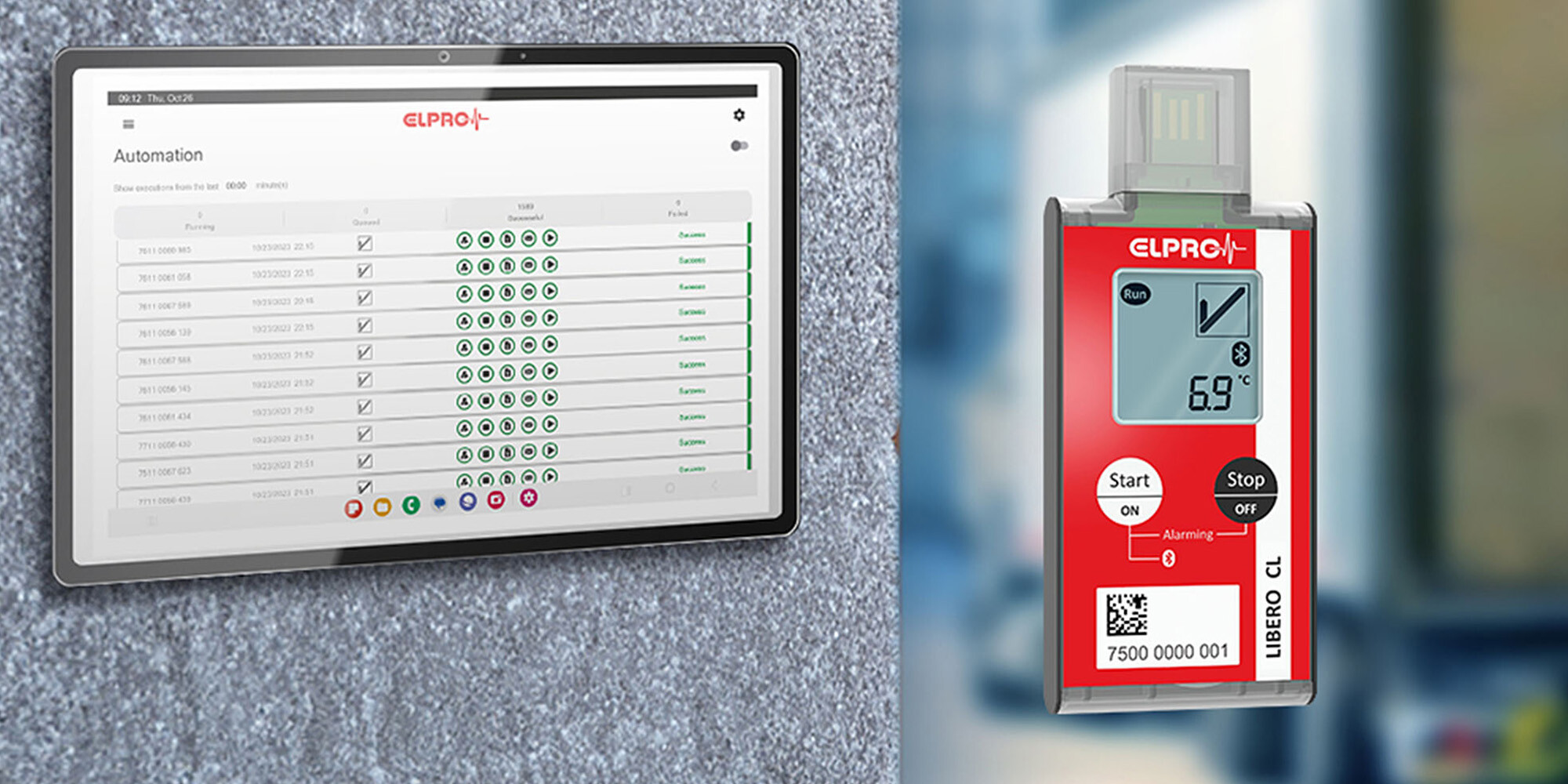
Multi-Use Bluetooth®
- Temperature and humidity
- USB or Bluetooth® connection
- Automated read-out of data or PDF

Cellular IoT Real-Time
- Temperature, humidity and position
- Real-time data generation and alarming
- Single-Use / Multi-Use
Monitoring Solutions for Every Temperature Range
Ambient
+15 °C to +25 °C
Used for standard pharmaceutical tablets, capsules and suitable for stable diagnostic kits; protects temperature-sensitive medical devices; also used for cosmetics requiring consistent conditions
Cool
+2 °C to +8 °C
Common in pharmaceuticals, vaccines, biologics, and injectable drugs like insulin; essential for gene/cell therapies; preserves supplements and probiotics; maintains diagnostic kit stability
Frozen
-20 °C to -80 °C
Essential for gene therapies, biologics, and viral vectors; preserves cell/tissue and stem cells; required for certain vaccines needing deep freeze; used for cryopreservation of biological materials
Dry Ice
-40 °C to -80 °C
Used for biologics and gene therapies; preserves viral vectors and cell-based treatments; critical for mRNA vaccines; maintains cell lines and various highly-sensitive biological samples
Cryogenic
-150 °C to -196 °C
Effectively suspends biological activity, prevents degradation and preserves biological function for extended periods. Protects cell/gene therapies, stem/CAR-T cells, tissue samples and more
Comparing our old process to the new one is like comparing a bike to a car. Both can cover a certain distance, but in terms of speed and comfort, they are incomparable. The new solution stands for long-term modernization, digitalization and entry into the modern world.
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk Cold Chain Distribution. Contact Us Today.

Common Questions About Cold Chain Logistics Monitoring Solutions
Regardless of the nature of your cold chain logistics application, you demand a reliable, compliant environmental monitoring solution that will scale and grow with your organization, protect your valuable products and ensure quality and data integrity from end-to-end. Below is a list of important questions ELPRO recommends you ask service providers when evaluating a solution.What is cold chain distribution in a supply chain?
There are many complex temperature monitoring scenarios involved when considering cold chain distribution in a supply chain. Several temperature-controlled scenarios for global delivery below may include the following data logger or monitoring device type:
- One way with product (single-use)
- Multi-step cold chain depots (multi-use)
- One way on product and cold room storage (single-use)
- End-to-end on product (box-level temperature monitoring indicators)
- Sample collection (box-level temperature monitoring indicators)
- One way with product and return (single-use or electronic temperature indicators)
- Continuous on mobile equipment (real-time on containers and/or transport vehicles)
What are supply chain qualification requirements?
For cold chain products and equipment used in transit, anything used in a GDP or GMP environment to store or transport products must be qualified. This applies to containers, cold boxes, airplanes, truck and vans fleets, and even trade lanes. Qualification is the process of proving that a piece of equipment, a room, a shipping lane, or even an entire network fulfils an intended purpose. Compared to qualifying equipment such as cryogenic shippers or freezers, and rooms, the qualification of an entire network is much more complex and requires significant analysis and process documentation.
Does the solution ensure real-time monitoring and alerting of temperature excursions?
If your application requires continuous monitoring, be certain to determine, if the IoT sensors continuously monitor all parameters and transmit data in real time via e-mail, SMS, or app notifications when a temperature deviation is detected, ensuring more time for rapid corrective action.
Is the environmental monitoring system compliant with GDP and GMP regulations?
If you require this, the solution must comply with GDP, GMP, be GAMP® 5 validated and comply with FDA 21 CFR Part 11. It ensures data integrity and proper temperature control to maintain regulatory compliance.
How is data integrity maintained, and does the system offer tamper-proof or auditable records?
The system must employ encrypted data transmission and secure cloud storage to ensure data integrity. It must also feature automated audit trails, so all system activities are logged and can be reviewed for compliance purposes.
Can the solution provide end-to-end visibility for temperature control across the entire cold chain (from manufacturing to transportation to storage)?
Intelligent database solutions such as ELPRO's liberoMANAGER and elproMONITOR provide comprehensive end-to-end visibility through cloud-based dashboards, offering real-time tracking of environmental parameters at every stage of the cold chain, whether in transit, at storage facilities, or during manufacturing.
What is the scalability and flexibility of the system as our needs grow or change?
The system you select should be highly scalable, capable of supporting additional sensors, warehouses, and geographies as your operations grow and expand, even globally. Be sure it can be configured to monitor multiple environmental parameters and integrate with other enterprise (ERP) business systems as your operations expand.
How does the system handle data logging and reporting, and can it generate customized compliance reports?
All data need to be logged securely and continuously. The system must be able to generate fully customized reports for compliance audits. Reports could include temperature history, excursion details, and corrective actions, formatted to meet regulatory requirements.
What backup or contingency measures are in place in case of system failure or connectivity issues?
In the event of system failure or lost connectivity, sensors and hardware need to buffer/store data locally and automatically sync with the cloud once connection is restored. Your system should also have redundant servers to prevent critical data loss and ensure continuity.
Does the system support integration with other enterprise software (e.g., warehouse management, transportation management, ERP systems)?
Environmental monitoring database solutions like elproMONITOR and ELPRO's liberoMANAGER are designed to integrate seamlessly with various enterprise platforms, allowing for centralized control and dashboard visibility across the entire supply chain.
What are the system’s capabilities for monitoring multiple environmental factors beyond temperature (e.g., humidity, light exposure, vibration)?
The cold chain logistics database solutions must support multi-parameter monitoring, including temperature, humidity, light exposure, tilt, vibration, with customizable alerts for each environmental factor to ensure comprehensive protection of valuable and sensitive products.
How does the solution handle the validation and calibration of sensors and devices?
The system must be validated and calibrated to meet the specified quality and ISO standards in your SOP. Calibration certificates and routine inspections ensure that hardware and sensors remain accurate and compliant with industry standards.
What is the system's user-friendliness, and how much training is required for staff to operate it effectively?
The system design and interface should be intuitive and user-friendly, with a simple features and dashboards for monitoring, conducting analyses, assessments and generating reports and archiving.
What is the expected response time and customer support availability in case of issues?
Customer support should be made available 24/7, offering rapid response times. Issues should typically be addressed within hours, via phone, email, text and online chat support. The environmental monitoring system should also feature remote alarming capabilities to immediately notify stakeholders of deviations via e-mail or SMS. The feature should also enable system manager to assign user groups to ensure redundancy.
What is the total cost of ownership (TOC), including installation, maintenance, and any hidden or recurring fees?
The initial cost of an intelligent cold chain logistics database and environmental monitoring solution includes hardware (sensors and data loggers), software, installation/setup. Optional costs may include onboard system training, project management and GxP services (GDP/GMP, qualification and temperature mapping, for example) to ensure compliance, if required. Ongoing costs are typically subscription-based software access, cloud storage, and maintenance. A detailed quote should outline all associated fees and services.
How does the system support GDP/GMP temperature mapping and qualification for storage and transportation facilities and equipment?
The cold chain logistics temperature monitoring solution should include services such as temperature mapping to document variability in specific storage or transport areas. It should also support qualification reports to demonstrate compliance with regulatory guidelines during facility audits.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


