Most biopharmaceutical products are subject to change as they age. Nevertheless, they remain stable as long as they retain their properties or potency. This stability period is also known as shelf life. Each product’s shelf life will vary depending on the data collected in the stability tests. Stability tests are the experimental protocols used for data collection serving as the basis for estimation of shelf life. Typical storage conditions of packaged pharmaceutical products, which are either used in hospitals or sold over-the-counter, are 2-8°C, 15-25°C, and sometimes 2-25°C.
Example: Results from accelerated stability testing—potency of a biopharmaceutical product at different temperatures and duration.
 Temperature Excursion Allowance: A GxP Compliant Solution
Temperature Excursion Allowance: A GxP Compliant Solution
By programming stability information into data loggers, you can prevent products from being discarded prematurely.
What is a Stability Budget?
The stability budget concept is a practical management tool to allocate to each supply chain partner for the manufacturing, packaging, storage, and distribution of biopharmaceutical products. The budget can apply to all pharmaceuticals, including those with storage conditions at -20°C, 2-8°C, and 15-25°C. The stability budget combines relevant information from temperature studies with available data from the stability testing to determine the amount of time a product can spend out of its specified storage conditions without risk to its quality, safety, or efficacy.
Throughout the product’s cycle, it will move through multiple “legs”’ in the supply chain. As there is always the risk of temperature excursions in each leg, a stability budget could help support the product’s safety at the end of the chain.
Furthermore, a stability budget prevents expensive, time-consuming investigations with contract research organizations (CRO) and logistics service providers (LSP). Delays can be avoided, fewer products will be discarded, and costs will be reduced. Most importantly, a stability budget can prevent drug shortages and ensure critical drugs reach patients in time.
How to Set Up a Stability Budget
Many pharmaceutical manufacturers use data from the extensive product stability studies to determine the minimum and maximum temperatures and time out of storage during transport.
Determining the product’s thermal stability is the first step. A product’s thermal stability is the product's ability to resist, under specified conditions, irreversible changes in its identity, strength, quality, and purity at various temperatures above and below the specified storage range.
As one can imagine, time is of the essence, especially when developing a new drug substance. Therefore, instead of solely relying on real-time stability tests that may take years, most pharmaceutical manufacturers also choose to conduct accelerated stability studies and predict the shelf life using the Arrhenius equation. In these accelerated tests, the products are exposed to defined sets of elevated temperatures (e.g., 25°C, 30°C, 40°C). The potency is tested after defined periods (e.g., six days, 28 days, three months, 12 months, 24 months, and 36 months). These simulations are analyzed to determine the impact temperature excursions have on drug degradation. The idea is to run the worst-case scenario.
In as much as a stability budget makes it possible to guarantee the product's safety and efficacy to the patient, success is only possible with the commitment of every partner involved in the supply chain.
Who Owns the Stability Budget?
The owner of the stability budget is the marketing authorization holder (MAH) – the company that applies for registration of a product in a certain market. Within this company, the natural owner is the quality department. From a document or data perspective, the stability budget is a defined set of data under strict version control, which is typically documented in a PDF document, sometimes also stored in dedicated software.
How Often Does a Stability Budget Change?
Stability budgets and expiration dates can increase over time but only in early phase I clinical trials for new drug substances. Stability budgets and expiration dates are typically set in phase II clinical trials. They remain stable for the duration of phase II as well as into commercial use. It is unlikely for the stability budget or the expiration date to change in later commercial phases. Expiration dates must be available in clear text on each kit as well as on the carton. For example, each vial needs to be labelled as well as the carton containing the vials.
How to Conduct a Stability Test
Stability tests can increase the time and temperature margin in which a pharmaceutical product can be exposed. During stability testing, the active ingredient is exposed to temperatures outside the specified storage condition for a certain time and afterwards tested to prove its safety and potency.
There are two types of stability testing:
- In real-time stability testing, a product is stored at recommended storage conditions and monitored until it fails the specification. One such tool is the mean kinetic temperature (MKT). This can be time consuming – and time is of the essence in product development.
- In accelerated stability tests, the product is stored at increased temperatures. After defined time periods, the product’s degradation is assessed and compared with the prediction of the known relationship between the acceleration factor (temperature) and the degradation rate (Arrhenius equation).
Is the Stability Budget Shared?
The stability budget is owned by the marketing authorization holder (MAH). They typically do not share the stability data with logistics providers (3PL, forwarders, wholesale) since they need to safeguard the product integrity up until patient delivery. Therefore, they purchase dedicated temperature-controlled services from their logistics service providers (LSP), which are typically refrigerated or room temperature where they will never exceed conditions like 2-8°C or 15-25°C. Coming up with rules that are more comprehensive (for example, 2-8°C, but it can have 10-hour excursions up to 20°C) would be much too complex for LSPs. However, if manufacturers control the stability budget and use it in an efficient way (e.g., input temperature limits into the data logger and/or the database), their workloads could be lighter, and they could save on costs.
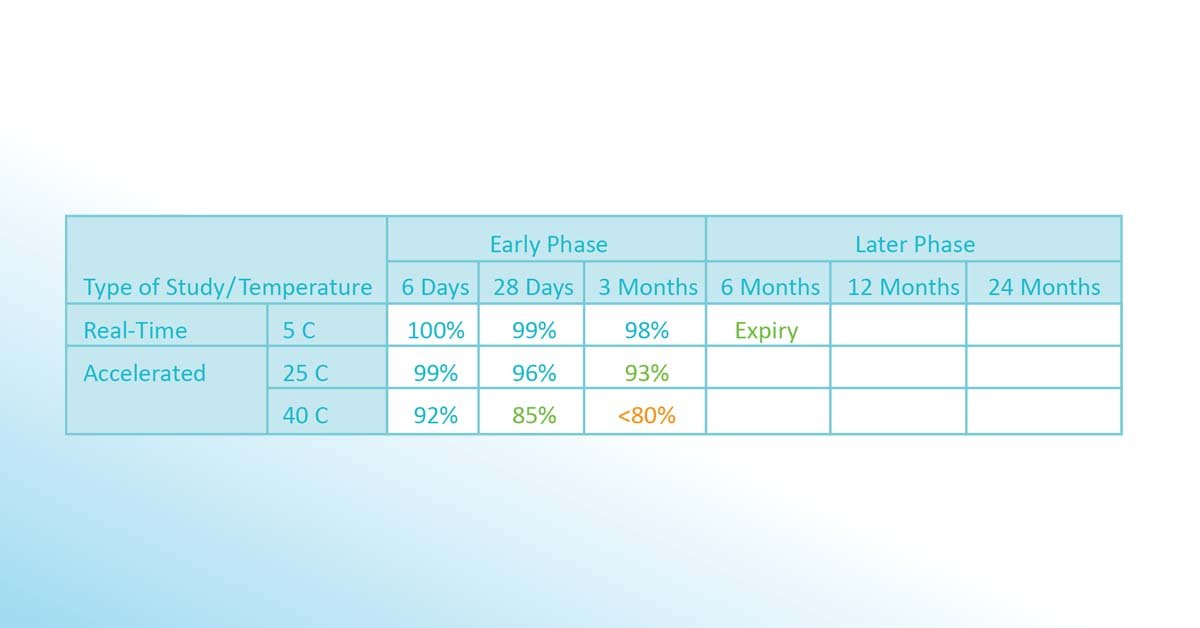
Different lots or batches of the substance are exposed to different temperatures. After different periods (6 days, 28 days, 3 months), a test is performed if more than 80% of the active ingredient is still potent. As a result of this early phase, a first version of the stability budget is issued (in above case, 8-25°C for 3 months, 25-40°C for 28 days). Since after 3 months, more than 98% of the active ingredient is potent, a first (conservative) version of the expiration date is calculated using the Arrhenius equation and defined to be 6 months.
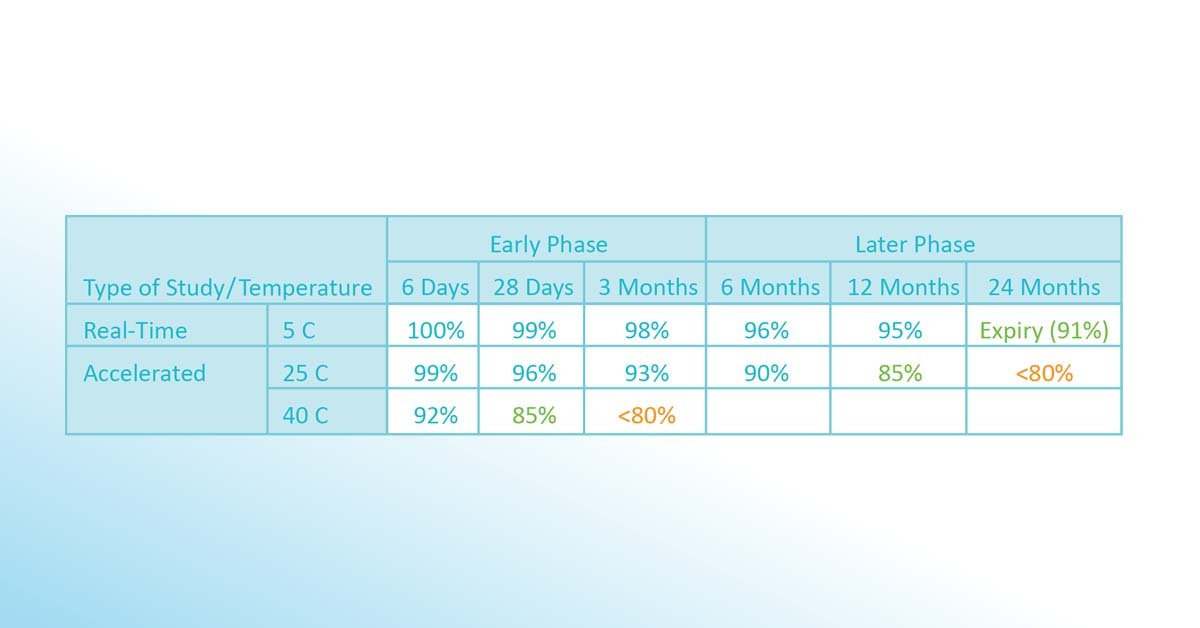
The same substance is still exposed to the same temperatures (and tested after different periods) to determine if the ingredient is still over 80% potent. As a result, a new version of the stability budget is issued (in above case, 8-25°C for 12 months and still 25-40°C for 28 days). After 24 months, still 91% of the potency is available if stored at 5°C. Based on stress tests and other safety and market considerations, the final expiration date is defined to be 24 months.
 Prepare for the Risks
Prepare for the Risks
Hear what the experts have to say regarding supply shortages, process changes, distribution failures, drug stability. These are a few concerns to address before you get started.
Let's Talk About Temperature Monitoring
Schedule a call with our experts today. We're here to support your cold chain monitoring project and help ensure it is successful.